A faster way to figure out what bacteria is causing a potentially deadly bloodstream infection could let doctors treat it more quickly and efficiently.
Category: Uncategorized
How powdered rock could help slow climate change
A method called enhanced rock weathering shows promise at capturing carbon dioxide from the air. But verifying the carbon removal is a challenge.
Something weird is happening to Earth’s inner core
A new study claims to confirm that the inner core is now rotating more slowly than it was over a decade ago, but some researchers remain skeptical.
Getting wild mosquitoes back to the lab alive takes a custom backpack
The new low-tech transportation method could help scientists in Africa assess if malaria-carrying mosquitoes are resistant to a common insecticide.
Will stashing more CO2 in the ocean help slow climate change?
Research is needed on how ocean carbon removal methods — such as iron fertilization and direct capture — could impact the environment.
A new method of making diamonds doesn’t require extreme pressure
Lab-grown diamonds can form at atmospheric pressure in a liquid of gallium, iron, nickel and silicon.
Tiny treadmills show how fruit flies walk
A method to force fruit flies to move shows the insects’ stepping behavior and holds clues to other animals’ brains and movement.
A decades-old mystery has been solved with the help of newfound bee species
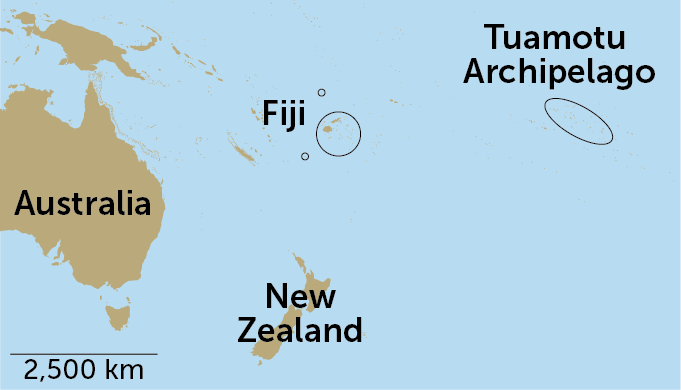
In 1965, renowned bee biologist Charles Michener described a new species of masked bee from “an entirely unexpected region,” the Tuamotu Archipelago of French Polynesia. Michener named the bee Hylaeus tuamotuensis and noted that its nearest relatives live in New Zealand — some 3,000 miles away across the Pacific Ocean. How did a small bee make such a big journey?
It turns out that the answer was buzzing above scientists’ heads all along. By swinging insect nets high up in the trees, researchers discovered eight species of Hylaeus bees that had never been described before, including six that live in Fiji.
The island nation lies between French Polynesia and Australia, where Hylaeus diversity is highest, so the scientists suspect that the ancestors of H. tuamotuensis reached their remote home by island-hopping across the Pacific. As individual bees moved from island to island, they steadily evolved into separate species, researchers report February 26 in Frontiers in Ecology and Evolution.
Most bee species live in arid regions like the southwestern United States, says bee biologist Bryan Danforth of Cornell University, who was advised by the late Michener for his Ph.D. “We don’t think of bees as being terribly diverse in islands.”
Bee searchers usually snag their quarry by sweeping nets low to the ground. But during a trip to Fiji in 2019, evolutionary biologist James Dorey of Wollongong University in Australia took a different approach. He knew that some bees in Australia tend to fly in the canopy of eucalypt trees, or gum trees, and thought bees in Fiji might do the same. He equipped himself with a longer net and started swinging it skyward.
“As soon as I was able to sample a flowering tree, we were catching Hylaeus,” Dorey says. “It was clear that we had more than one [new] species from that one tree.”
Searching for bees in treetops is relatively rare. But “we’re starting to realize that, actually, there’s a lot of bee diversity up there,” Danforth says.

Dorey and his collaborators have a strong relationship with local Fijians, especially in Navai Village on the main island of Viti Levu, and with Fijian scientists like coauthor Marika Tuiwawa, a botanist at the University of the South Pacific. There is a lot of enthusiasm among Fijians for their native bees, Dorey says, and he hopes to train students there in bee collecting. Experts on Fiji’s bees, he says, should be Fijian.
While it’s clear that H. tuamotuensis is not alone in its remote island home, many mysteries remain: How did Hylaeus bees make it to the various islands and what path did they take?
It’s possible the bees were blown across the Pacific by storms, Danforth says, but he also thinks that their habit of nesting in wood may have something to do with the bees’ spread. “If you nest in wood and a piece of wood falls in the ocean, and that drifts thousands of kilometers and lands on a habitable spot, that’s a plausible way for these bees to disperse,” he says. “We know that other wood-nesting bees have done that.”
Four years on, the COVID-19 pandemic has a long tail of grief
March 11 marks the fourth anniversary of the World Health Organization’s declaration that the COVID-19 outbreak was a pandemic. COVID-19 hasn’t gone away, but there have been plenty of actions that suggest otherwise.
In May 2023, WHO announced COVID-19 was no longer a public health emergency (SN: 5/5/23). The United States shortly followed suit, which meant testing and treatments were no longer free (SN: 5/4/23). And on March 1 of this year, the U.S. Centers for Disease Control and Prevention loosened their isolation guidelines for people with COVID-19. Now the CDC says infected people can be around others as soon as a day after a fever subsides and symptoms are improving, even though someone is contagious during an infection for six to eight days, on average (SN: 7/25/22).
These outward signs of leaving the pandemic chapter behind neglect to acknowledge how many people cannot (SN: 10/27/21). Nearly 1.2 million people have died in the United States from COVID-19. Close to 9 million adults have long COVID. Nearly 300,000 children have lost one or both parents.
There has been little official recognition in the United States of the profound grief people have experienced and continue to experience. There is no federal monument to honor the dead — mourners have constructed their own memorials. A resolution to commemorate the first Monday of March as “COVID-19 Victims Memorial Day” awaits action by the U.S. Congress.
Many people are coping not just with the deaths of family and friends from COVID-19, but with how the pandemic robbed them of the chance to say goodbye to loved ones and grieve with their family and community. Researchers are studying the extent to which these losses rippled out into society and how the pandemic interrupted the grieving process.
Emily Smith-Greenaway, a demographer at the University of Southern California in Los Angeles, was part of team that estimated that for every one COVID-19 death, there are nine bereaved family members (SN: 4/4/22). Sarah Wagner, a social anthropologist at George Washington University in Washington, D.C., leads a project called Rituals in the Making, which is examining how the pandemic disrupted rituals and the experience of mourning through interviews with mourners and death care workers, among other research methods. Science News spoke with Smith-Greenaway and Wagner about their work. The interviews have been edited for length and clarity.
SN: Why is it important to estimate the number of close family members affected by COVID-19 deaths?
Smith-Greenaway: We typically quantify mortality events in terms of numbers of casualties. By shedding light explicitly on the concentric circles of people surviving each of the deaths, we offer a much more experiential perspective — the burden that a large-scale mortality event imposes on those who are still alive. It also allows us to kind of rescale the true sense of the magnitude of the crisis.
[With the number of deaths today,] our model demonstrates that about 10.5 million people have lost a close relative to COVID, [which includes] grandparents, parents, siblings, spouses and children. We’re not even capturing cousins, aunts, uncles. Think about how many children lost teachers or how many neighbors or friends or coworkers [died]. This is an underestimate when we’re thinking about the many people who are affected by each single death.
SN: What motivated the Rituals in the Making project?
Wagner: We began in May of 2020, and this was this period of heightened pandemic restriction and confinement. We posed what we saw as a fundamental question: How do we mourn when we cannot gather? Particularly in that first year, we were focused on the rituals around funeral, burial and commemorative practice and how they would be impacted and changed by the pandemic. In the last two years, [the project] has included the ways in which misinformation also compounds individual grief and more collective mourning.
A throughline in the research is that this mourning was interrupted and constrained by the conditions of the pandemic itself, but also troubled by politicization of the deaths. And then [there’s] this expectation that we move on, we push past the pandemic, and yet we have not acknowledged the enormity of the tragedy.
SN: Why are rituals and memorials important to grieving?
Wagner: We think about rituals as providing a means to respond to rupture. We are able to come together, gathering to stand before a coffin to say goodbye, or to have a wake, to sit down and have a meal with the bereaved. They are about providing an opportunity to remember and honor that loved one. But they are also about the living — a way of supporting the surviving family members, a way of helping them out of the chasm of that grief.
Memorials [such as a day of remembrance or a monument] are a nation saying, we recognize these lives and we anoint them with a particular meaning. We think about memorials as forms of acknowledgement and a way of making sense of major tragedies or major sacrifices.
In the context of the pandemic, the rituals that are broken and [the lack of] memorials at that national level help us see that the mourners have been left in many ways to take memory matters into their own hands. The responsibility has been pushed onto them at these acute moments of their own grief.
SN: How has the pandemic impacted survivors and the grieving process?
Smith-Greenaway: Societies have demographic memory. There is a generational effect any time we have a mortality crisis. A war or any large-scale mortality event lingers in the population, in the lives and memories of those who survived it.
This pandemic will stay with us for a very long time. [There are] young people who remember losing their grandma, but they couldn’t go see her in the hospital, or remember losing a parent in this sudden way because they brought COVID-19 home from school. So many lives were imprinted at such an early stage of life.
Wagner: Whether we are talking to the bereaved, members of the clergy, health care workers or staff from funeral homes, people describe the isolation. It is incredibly painful for families because they weren’t able to be with their loved one, to be able to touch someone, to hold their hand, to caress a cheek. People were left to wonder, “was my loved one aware? Were they confused? Were they in pain?” [After the death], not being able to have people into one’s home, not being able to go out. That sort of joy of having other people around you in your depths of grief — that was gone.
As the study progressed, [we learned about] the impact political divisiveness had on people’s grief. [Families were asked,] did the person have underlying health issues? What was the person’s vaccination status? It was as if the blame was getting shifted onto the deceased. Then to be confronted with, “this is all just a hoax,” or “[COVID-19 is] nothing worse than a bad cold.” To be a family member, and to struggle for recognition in the face of these conversations that their loved ones’ death and memory is not just dismissed, but in a way feels denied.
SN: How can society better support the need to grieve?
Smith-Greenaway: Bereavement policies are not very generous, as we would expect in America. Sometimes it’s one, two or three days. They’re also very restrictive, where it has to be a particular relation.
Think about kids. I’m a professor at a university. There’s this callous joke that college students just tell you their grandmother died because they don’t want to turn something in. This reflects how we treat bereavement as a society, especially for young people. Kids’ grief can often be misunderstood. It’s perceived to be bad behavior, that they’re acting out. I think we need comprehensive school policies that take better care to recognize how many kids are suffering losses in their lives.
Wagner: We’re enveloped in this silence around pandemic death. I think there’s a willingness to talk about the pandemic losses in other realms, the economic losses or the loss of social connection. Why is there this silence around 1.2 million deaths — the enormity of the tragedy?
If you know someone who has lost a loved one to COVID-19, talk to them about it. Ask them about that loved one. Just being an active part of conversations around memory can be a beautiful act. It can be a restorative act.
The blood holds clues to understanding long COVID
When I talk to immunologist Paul Morgan, he’s on the hunt for potentially life-altering drugs.
He’s got a call with a pharmaceutical company planned in the next half hour. His goal: persuade the company to supply his lab with a drug that might — maybe, hopefully, someday — ease some of the unrelenting symptoms of long COVID.
Morgan’s lab at Cardiff University in Wales has been studying people with the disease, including the first waves of patients, some of whom have been living with long COVID for more than two years (SN: 8/21/23). The “very long haulers,” he calls them. Their symptoms can include brain fog, fatigue, breathlessness and joint and muscle pain.
Morgan and his colleagues have pinpointed an immune system anomaly in the blood of some of these long haulers. A drug that targets that quirk might be one way to treat their disease. He’s quick to tell me that this research is in its early days. First, his team needs to get its hands on a drug. Then they need to do a clinical trial — at this stage, it’s still proof-of-concept. “Actual treatments are still some way off,” he says.
But the work is one in a surge of studies mining the blood for long COVID clues, potential biomarkers of the disease. A growing cadre of labs are starting to sketch out some of the shrouded figures at play in the once-seemingly inscrutable disease. It’s as if there’s a “picture that’s being revealed from the fog,” says Akiko Iwasaki, an immunologist and Howard Hughes Medical Institute investigator at Yale University.
That picture features a motley cast of molecular and cellular characters that could point scientists toward potential tests and treatments — both are currently lacking. Still, the full long COVID landscape is unquestionably complicated. Assorted actors tangle together in an immunological thicket that can make the disease seem impenetrable.
But Iwasaki thinks researchers can crack long COVID’s mysteries. In fact, recent work in the field has her feeling pretty excited. Yes, investigating long COVID is complex, she says. “But it’s definitely worth doing because many people are suffering.”
I spoke with Iwasaki, Morgan, and other researchers about long haulers and current work to illuminate the disease — and to get clarity on the less severe but still vexing symptoms that stuck around for months after my own COVID-19 infection.
Long COVID is not a single disease, which makes diagnosis and treatment difficult
Some 5 to 20 percent of COVID-19 cases develop into long COVID, though the exact number is hard to pin down. One formidable challenge is diagnosis. Does a person with bone-deep exhaustion and trouble concentrating on the pages of their book have the disease? There’s no test or nasal swab doctors can use to provide a definitive answer.
For diseases like diabetes and prostate cancer, scientists look for biomarkers in the blood — molecules that can serve as telltale signs of illness. But “we are not likely to come up with one biomarker or even one set of biomarkers to distinguish everyone with long COVID,” Iwasaki says.
The difficulty is that long COVID is not just one disease. It’s likely a collection of many diseases, she says, with varying sets of symptoms and triggers. Even defining long COVID is complicated. Last spring, researchers developed a long COVID scoring system that factors in a dozen signature symptoms. But more than 200 symptoms have been tied to the disease, and cases can vary greatly from person to person (SN: 11/17/22).
Today, in many doctors’ offices and clinics, homing in on a diagnosis means first ruling out other conditions. For example, after months of feeling wrung out and faded, with unexpected pain in my legs following a COVID-19 infection last year, my doctor ordered a slew of blood tests. She was looking for signs of rheumatoid arthritis, Lyme disease, thyroid hormone abnormalities and a handful of other conditions. Everything was negative. It’s called a “diagnosis of exclusion,” Morgan says. “You test for everything else, and when nothing comes back positive, you say, ‘well, it must be [long COVID], then.’”
He compares it to myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, another debilitating condition that also lacks clear diagnostic tests. “Many physicians don’t take it very seriously at all because of that.” That concern is even listed on the U.S. Centers for Disease Control and Prevention’s ME/CFS Web page. And it’s true of long COVID, too, says Wolfram Ruf, an immunologist at University Medical Center Mainz in Germany. “There’s still some misconception that this is just a psychological disease.” A 2022 survey found that a third of long COVID patients felt as if medical professionals dismissed their illness. And stories from frustrated long haulers continue to appear in the news.
Better diagnostics mean people suffering from long COVID’s myriad symptoms could put a firm label on their condition. That’s important, Morgan says. But tests that spot long COVID biomarkers in the blood could also do far more. “If those tests actually lead you to a mechanism and to a treatment,” he says, “then that’s transformational.”
A complicated cast of immune and other characters may contribute to long COVID
Morgan’s lab and others have zeroed in on immune proteins that defend us from bacteria and viruses. These proteins, part of a defense called complement, circulate in the blood, and get chopped up during an infection. The resulting fragments sound a “we’re under attack” alarm and help form a molecular machine that busts pathogens.
Once the infection clears, the fragments fade away. But in some people with long COVID, the alarm-raising fragments can linger in the blood, Morgan’s team reported February 14 in Med. That’s a sign that the defense system is still whipping up inflammation — in some cases, even years after a person’s initial COVID-19 infection. In January, a different group reported something similar in Science in a study of 113 COVID-19 patients.
Not everyone with long COVID will have complement abnormalities. Even in the patients Morgan studies, “there will be some people who have no changes in their complement markers at all.”
But for those who do, it can be a double-edged sword, he says. Turning complement on briefly can knock out some bugs, but keeping it on chronically can damage your cells.
There’s an ever-growing list of other blood-borne anomalies, too. A protein found in the brain can leak out into the blood in people with brain fog, scientists found in February. And long COVID patients can have low levels of the stress hormone cortisol, Iwasaki’s team reported in Nature in September. In some patients, she and others have also spotted other suspicious signs, like long-slumbering herpes viruses that reawaken and start infecting cells again. “Whether this is a cause or effect [of long COVID],” Iwasaki says, “we don’t know.” Usually, the immune system keeps these viruses under control.
In some people with long COVID, powerful immune players called T cells also seem to go out of whack. Scientists in England analyzing the blood of long COVID patients found T cells that release high levels of an alarm signal called interferon-gamma. That signal could serve as a potential biomarker in some patients, the study’s authors suggested.
“Perhaps there’s SARS-CoV-2 somewhere in the body that can’t be cleared.”
Nadia Roan
At the University of California, San Francisco, virologist and immunologist Nadia Roan’s team is also taking a look at T cells. In some long COVID patients, T cells that recognize SARS-CoV-2 can become exhausted, her team reported in Nature Immunology in January. And scientists know that tired-out T cells can have trouble wiping out infection.
“Perhaps there’s SARS-CoV-2 somewhere in the body that can’t be cleared,” Roan says. If a hidden reservoir of virus lurks in people’s tissues long-term, certain T cells might gather there for continued attack, eventually wearing the cells down. Last summer, a different team found viral traces in some people’s guts nearly two years after their initial infection. Scientists have also spotted signs of SARS-CoV-2 in other body tissues, including brain, lung and liver. These traces may be enough to irritate the immune system long term.
Residual virus isn’t likely to explain everything. “Different mechanisms may drive different forms of the disease,” Roan says. But together, work from her lab and others paints a portrait of an immune system under enduring duress. And as the pictures comes into focus, scientists are starting to explore new experiments, trials and treatments.
Clinical trials will test whether antiviral drugs can ease long COVID symptoms
Iwasaki and her colleagues are nearly done recruiting 100 people with long COVID for a randomized clinical trial of the antiviral drug Paxlovid. A similar trial by the National Institutes of Health is also in the works. That study aims to enroll 900 people and should conclude testing by this summer.
An idea targeted by both trials is that SARS-CoV-2 persists in the body, triggering symptoms. Scientists will follow participants as they take Paxlovid for 15 or 25 days. (For COVID-19 infection, the typical treatment length is five days.)

Iwasaki’s team also plans to scan participants’ blood for molecules that might predict a person’s response to Paxlovid. Suppose participants who improve after Paxlovid had high levels of certain blood molecules prior to treatment. Scientists could then measure those molecules in other people with long COVID to see if they might be good candidates for the drug. “There’s not one drug cure-all,” Iwasaki says, “but even knowing who might benefit is a huge thing.”
And though Morgan’s complement work is still at an early stage, he considers it a “strong lead to a potential therapy.” One bright spot is the number of complement-targeting drugs that already exist. Doctors currently use the drugs to treat certain blood disorders and other rare conditions. Morgan and other scientists have tried using some of these drugs to treat severe cases of acute COVID-19 infection, but large-scale trials didn’t pan out.
Now, he wants to repurpose those drugs for long COVID patients whose complement system has gone out of control. Dialing down those defenses might help quench the fire of chronic inflammation.
So far, Morgan hasn’t seen much interest from pharmaceutical companies. Repurposing generic drugs isn’t a big money maker. And Morgan’s team doesn’t envision long COVID patients needing to use the drugs long-term — another financial disincentive for companies. But when I email him later, he says his call after our interview went well. Morgan’s not naming the company yet but he knows that patients are standing by, waiting day after day for anything that will offer some relief.
For me, lingering symptoms turned the once-easy tasks of everyday life into energy-sucking feats performed while my body’s battery blinked down to zero. If I miscalculated and walked too fast or moved too much, I’d pay for it later and crash on the couch or in bed, sometimes taking days to recover. That symptom is known as post-exertional malaise and it’s common for people with long COVID.
But I was never officially diagnosed with the disease. Keeping up with doctors’ appointments felt daunting, and I wasn’t sure how much my doctor would be able to help. There may be many people who fall into this category, Morgan says — people who have long-term symptoms but lack clear-cut answers.
Though white women like myself are most likely to be diagnosed with the disease, in a survey from the U.S. Census Bureau, Hispanic and Black people were more likely to report long-lasting symptoms. Access to care could be one factor in the diagnosis discrepancy. Previous data have shown that people from these groups are less likely to have health insurance. Another factor may be the changeling nature of the disease itself. Long COVID can show up differently in different groups of people, scientists have found.
I’m lucky because my symptoms started improving after about six months. I knew I was mostly recovered the first time I went grocery shopping and carried my bags into the house by myself, without crashing afterwards. But so many others continue to struggle.
Morgan sees that firsthand. Since his paper came out, “I’ve been absolutely deluged by letters from patients,” he says. They ask to be tested and if he has drugs that can treat them. For Morgan, those letters show the depth of the problem.
“I reply to them all,” he says.