To err is human, which is really not a very good excuse.
And to err as a scientist is worse, of course, because depending on science is supposed to be the best way for people to make sure they’re right. But since scientists are human (most of them, anyway), even science is never free from error. In fact, mistakes are fairly common in science, and most scientists tell you they wouldn’t have it any other way. That’s because making mistakes is often the best path to progress. An erroneous experiment may inspire further experiments that not only correct the original error, but also identify new previously unsuspected truths.
Still, sometimes science’s errors can be rather embarrassing. Recently much hype accompanied a scientific report about the possibility of life on Venus. But instant replay review has now raised some serious concerns about that report’s conclusion. Evidence for the gas phosphine, a chemical that supposedly could be created only by life (either microbes or well-trained human chemists), has started to look a little shaky. (See the story by well-trained Science News reporter Lisa Grossman.)
While the final verdict on phosphine remains to be rendered, it’s a good time to recall some of science’s other famous errors. We’re not talking about fraud here, or just bad ideas that were worth floating but flopped instead, or initial false positives due to statistical randomness. Rather, let’s just list the Top 10 erroneous scientific conclusions that got a lot of attention before ultimately getting refuted. (With one exception, there will be no names, for the purpose here is not to shame.)
10. A weird form of life
A report in 2010 claimed that a weird form of life incorporates arsenic in place of phosphorus in biological molecules. This one sounded rather suspicious, but the evidence, at first glance, looked pretty good. Not so good at second glance, though. And arsenic-based life never made it into the textbooks.
9. A weird form of water
In the 1960s, Soviet scientists contended that they had produced a new form of water. Ordinary water flushed through narrow tubes became denser and thicker, boiled at higher than normal temperatures and froze at much lower temperatures than usual. It seemed that the water molecules must have been coagulating in some way to produce “polywater.” By the end of the 1960s chemists around the world had begun vigorously pursuing polywater experiments. Soon those experiments showed that polywater’s properties came about from the presence of impurities in ordinary water.
8. Neutrinos, faster than light
Neutrinos are weird little flyweight subatomic particles that zip through space faster than Usain Bolt on PEDs. But not as fast as scientists claimed in 2011, when they timed how long it took neutrinos to fly from the CERN atom smasher near Geneva to a detector in Italy. Initial reports found that the neutrinos arrived 60 nanoseconds sooner than a beam of light would. Faster-than-light neutrinos grabbed some headlines, evoked disbelief from most physicists and induced Einstein to turn over in his grave. But sanity was restored in 2012, when the research team realized that a loose electrical cable knocked the experiment’s clocks out of sync, explaining the error.
7. Gravitational waves from the early universe
All space is pervaded by microwave radiation, the leftover glow from the Big Bang that kicked the universe into action 13.8 billion years ago. A popular theory explaining details of the early universe — called inflation — predicts the presence of blips in the microwave radiation caused by primordial gravitational waves from the earliest epochs of the universe.
In 2014, scientists reported finding precisely the signal expected, simultaneously verifying the existence of gravitational waves predicted by Einstein’s general theory of relativity and providing strong evidence favoring inflation. Suspiciously, though, the reported signal was much stronger than expected for most versions of inflation theory. Sure enough, the team’s analysis had not properly accounted for dust in space that skewed the data. Primordial gravitational waves remain undiscovered, though their more recent cousins, produced in cataclysmic events like black hole collisions, have been repeatedly detected in recent years.
6. A one-galaxy universe
In the early 20th century, astronomers vigorously disagreed on the distance from Earth of fuzzy cloudlike blobs shaped something like whirlpools (called spiral nebulae). Most astronomers believed the spiral nebulae resided within the Milky Way galaxy, at the time believed to comprise the entire universe. But a few experts insisted that the spirals were much more distant, themselves entire galaxies like the Milky Way, or “island universes.” Supposed evidence against the island universe idea came from measurements of internal motion in the spirals. It would be impossible to detect such motion if the spirals were actually way far away. But by 1924, Edwin Hubble established with certainty that at least sone of the spiral nebulae were in fact island universes, at vast distances from the Milky Way. Those measurements of internal motion were difficult to make — and they just turned out to be wrong.
5. A supernova’s superfast pulsar
Astronomers rejoiced in 1987 when a supernova appeared in the Large Magellanic Cloud, the closest such stellar explosion to Earth in centuries. Subsequent observations sought a signal from a pulsar, a spinning neutron star that should reside in the middle of the debris from some types of supernova explosions. But the possible pulsar remained hidden until January 1989, when a rapidly repeating radio signal indicated the presence of a superspinner left over from the supernova. It emitted radio beeps nearly 2,000 times a second — much faster than anybody expected (or could explain). But after one night of steady pulsing, the pulsar disappeared. Theorists raced to devise clever theories to explain the bizarre pulsar and what happened to it. Then in early 1990, telescope operators rotated a TV camera (used for guiding the telescope) back into service, and the signal showed up again — around a different supernova remnant. So the supposed signal was actually a quirk in the guide camera’s electronics — not a message from space.
4. A planet orbiting a pulsar
In 1991, astronomers reported the best case yet for the existence of a planet around a star other than the sun. In this case, the “star” was a pulsar, a spinning neutron star about 10,000 light-years from Earth. Variations in the timing of the pulsar’s radio pulses suggested the presence of a companion planet, orbiting its parent pulsar every six months. Soon, though, the astronomers realized that they had used an imprecise value for the pulsar’s position in the sky in such a way that the signal anomaly resulted not from a planet, but from the Earth’s motion around the sun.
3. Age of Earth
In the 1700s, French naturalist Georges-Louis Leclerc, Comte de Buffonestimated an Earth age of about 75,000 years, while acknowledging it might be much older. And geologists of the 19th century believe it to be older still — hundreds of millions of years or more — in order to account for the observation of layer after layer of Earth’s buried history. After 1860, Charles Darwin’s new theory of evolution also implied a very old Earth, to provide time for the diversity of species to evolve. But a supposedly definite ruling against such an old Earth came from a physicist who calculated how long it would take an originally molten planet to cool. He applied an age limit of about 100 million years, and later suggested that the actual age might even be much less than that. His calculations were in error, however — not because he was bad at math, but because he didn’t know about radioactivity.
Radioactive decay of elements in the Earth added a lot of heat into the mix, prolonging the cooling time. Eventually estimates of the Earth’s age based on rates of radioactive decay (especially in meteorites that formed around the same time as the Earth) provided the correct current age estimate of 4.5 billion years or so.
2. Age of the universe
When astronomers first discovered that the universe was expanding, at the end of the 1920s, it was natural to ask how long it had been expanding. By measuring the current expansion rate and extrapolating backward, they found that the universe must be less than 2 billion years old. Yet radioactivity measurements had already established the Earth to be much older, and it was very doubtful (as in impossibly ridiculous) that the universe could be younger than the Earth. Those early calculations of the universe’s expansion, however, had been based on distance measurements relying on Cepheid variable stars.
Astronomers calculated the Cepheids’ distances based on how rapidly their brightness fluctuated, which in turn depended on their intrinsic brightness. Comparing intrinsic brightness to apparent brightness provided a Cepheid’s distance, just as you can gauge the distance of a lightbulb if you know its wattage (oh yes, and what kind of lightbulb it is). It turned out, though, that just like lightbulbs, there is more than one kind of Cepheid variable, contaminating the expansion rate calculations. Nowadays converging methods give an age of the universe of 13.8 billion years, making the Earth a relative newcomer to the cosmos.
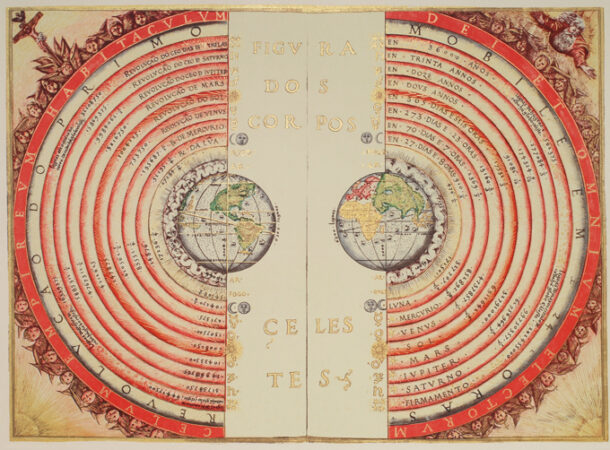
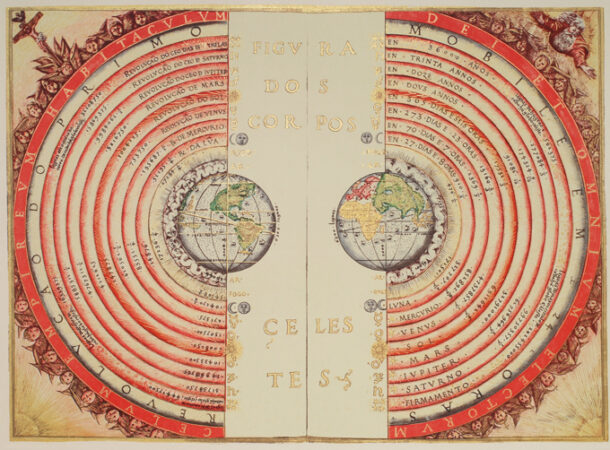
1. Earth in the middle
OK, we’re going to name and blame Aristotle for this one. He wasn’t the first to say that the Earth occupies the center of the universe, but he was the most dogmatic about it, and believed he had established it to be incontrovertibly true — by using logic. He insisted that the Earth must be in the middle because earth (the element) always sought to move toward its “natural place,” the center of the cosmos. Even though Aristotle invented formal logic, he apparently did not notice a certain amount of circularity in his argument. It took a while, but in 1543 Copernicus made a strong case for Aristotle being mistaken. And then in 1610 Galileo’s observation that Venus went through a full set of phases sealed the case for a sun-centered solar system.
Now, it would be nice if there were a lesson in this list of errors that might help scientists do better in the future. But the whole history of science shows that such errors are actually unavoidable. There is a lesson, though, based on what the mistakes on this list have in common: They’re all on a list of errors now known to be errors. Science, unlike certain political philosophies and personality cults, corrects its mistakes. That’s the lesson, and that’s why respecting science is so important to avoiding errors in other realms of life.