It feels good to recycle. There’s a certain sense of accomplishment that comes from dutifully sorting soda bottles, plastic bags and yogurt cups from the rest of the garbage. The more plastic you put in that blue bin, the more you’re keeping out of landfills and the oceans, right?
Wrong. No matter how meticulous you are in cleaning and separating your plastics, most end up in the trash heap anyway.
Take flexible food packages. Those films contain several layers of different plastics. Because each plastic has to be recycled separately, those films are not recyclable. Grocery bags and shrink wrap are too flimsy, prone to getting tangled up with other materials on a conveyor belt. The polypropylene in yogurt cups and other items doesn’t usually get recycled either; recycling a hodgepodge of polypropylene produces a dark, smelly plastic that few manufacturers will use.
Only two kinds of plastic are commonly recycled in the United States: the kind in plastic soda bottles, polyethylene terephthalate, or PET; and the plastic found in milk jugs and detergent containers — high-density polyethylene, or HDPE. Together, those plastics make up only about a quarter of the world’s plastic trash, researchers reported in 2017 in Science Advances. And when those plastics are recycled, they aren’t good for much. Melting plastic down to recycle changes its consistency, so PET from bottles has to be mixed with brand-new plastic to make a sturdy final product. Recycling a mix of multicolored HDPE pieces creates a dark plastic good only for making products like park benches and waste bins, in which properties like color don’t matter much.
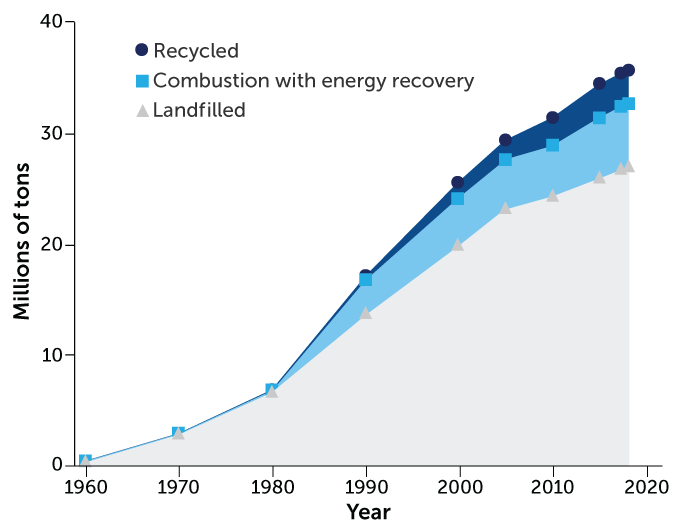
The difficulties of recycling plastic into anything manufacturers want to use is a big reason why the world is littered with so much plastic waste, says Eric Beckman, a chemical engineer at the University of Pittsburgh. In 2018 alone, the United States landfilled 27 million tons of plastic and recycled a mere 3 million, according to the U.S. Environmental Protection Agency. Low recycling rates aren’t just a problem in the United States. Of the 6.3 billion tons of plastic that have been discarded around the world, only about 9 percent has gotten recycled. Another 12 percent has been burned, and almost 80 percent has piled up on land or in waterways.
With plastic collecting everywhere from the top of Mount Everest to the bottom of the Mariana Trench, there’s an urgent need to reduce the amount of plastic that gets thrown away (SN: 1/16/21, p. 5). Some people propose replacing plastics with biodegradable materials, but those replacements are generally not as strong or cheap to make as plastics (SN: 6/22/19, p. 18). Since, realistically, plastic is not going away any time soon, chemists who understand the ins and outs of all this pesky plastic are working to make it easier to recycle and turn into higher-quality material that’s useful for more things.
“There’s not going to be a single technology that’s going to be the answer,” says Ed Daniels, senior project manager at the REMADE Institute in West Henrietta, N.Y., which funds research into new recycling techniques. Some projects are on the brink of breaking into industry; others are still just promising lab experiments. But all are focused on designing a future where any plastic that ends up in the recycling bin can have a second and third life in a new product.
Picking plastics apart
One of the biggest bottlenecks in plastic recycling is that every material has to get processed separately. “Most plastics are like oil and water,” says chemist Geoffrey Coates of Cornell University. They just don’t mix. Take, for example, a polyethylene detergent jug and its polypropylene cap. “If you melt those down, and I make a bottle out of that, and I squeeze it, it would basically crack down the side,” Coates says. “It’s crazy brittle. Totally worthless.”
That’s why the first destination for plastic recyclables is a material recovery facility, where people and machines do the sorting. Separated plastics can then be washed, shredded, melted and remolded. The system works well for simple items like soda bottles and milk jugs. But not for items like deodorant containers — where the bottle, crank and cap could all be made of different kinds of plastic. Food packaging films that contain several layers of different plastic are particularly tricky to take apart. Every year, 100 million tons of these multilayer films are produced worldwide. When thrown away, those plastics go to landfills, says chemical engineer George Huber of the University of Wisconsin–Madison.

To tackle that problem, Huber and colleagues devised a strategy for dealing with complex mixtures of plastics. The process uses a series of liquid solvents to dissolve individual plastic components off a product. The trick is choosing the right solvents to dissolve only one kind of plastic at a time, Huber says.
The team tested the technique on a packaging film that contained polyethylene and PET, as well as a plastic oxygen barrier made of ethylene vinyl alcohol, or EVOH, that keeps food fresh.
Stirring the film into a toluene solvent first dissolved the polyethylene layer. Dunking the remaining EVOH-PET film in a solvent called DMSO stripped off the EVOH. The researchers then plucked out the remaining PET film and recovered the other two plastics from their separate solvents by mixing in “antisolvent” chemicals. Those chemicals caused the plastic molecules that were dispersed in the liquids to bunch together into solid clumps that could be fished out.
This process recovered practically all of the plastic from the original film, the researchers reported last November in Science Advances. When tested on a jumble of polyethylene, PET and EVOH beads, the solvent washes recovered more than 95 percent of each material — hinting that these solvents could be used to strip plastic components off bulkier items than packaging films. So in theory, recovery facilities could use this technique to disassemble multiplastic deodorant containers and other products of various shapes and sizes.
Huber and colleagues next plan to look for solvents to dissolve more kinds of plastic, such as the polystyrene in Styrofoam. But it will take a lot more work to make this strategy efficient at sorting all the intricate plastic combinations in real-world recyclables.
Making plastics mix
There may also be chemical shortcuts that allow multilayer films and other mixtures of plastics to be recycled as they are. Additives called compatibilizers help different melted-down plastics blend, so that unsorted materials can be treated as one. But there is no universal compatibilizer that allows every kind of plastic to be mixed together. And existing compatibilizers are not widely used because they are not very potent — and adding a lot of compatibilizer to a plastic blend gets expensive.
To boost viability, Coates and colleagues created a highly potent compatibilizer for polyethylene and polypropylene. Together, those two plastics make up more than half of the world’s plastic. The new compatibilizer molecule contains two segments of polyethylene, interspersed with two segments of polypropylene. Those alternating segments latch onto plastic molecules of the same kind in a mixture, bringing polyethylene and polypropylene together. It’s as if polyethylene were made of Legos, and polypropylene were made of Duplos, and the researchers made a special building block with connectors that fit both types of blocks.
Having two polyethylene and two polypropylene connectors for each compatibilizer molecule, rather than one, made this compatibilizer stronger than previous versions, Coates and colleagues reported in 2017 in Science. The first test of the new compatibilizer involved welding together strips of polyethylene and polypropylene. Ordinarily, the two materials easily peel apart. But with a layer of compatibilizer between them, the plastic strips broke, rather than the compatibilizer seal, when pulled apart.
In a second test, the researchers mixed the compatibilizer into a melted blend of polyethylene and polypropylene. It took only 1 percent compatibilizer to create a tough new plastic.
“These are crazy potent additives,” Coates says. Other compatibilizers had to be added at concentrations up to 10 percent to hold these two plastics together. The new compatibilizer is now the basis for Coates’ start-up, Intermix Performance Materials, based in Ithaca, N.Y.
Good as new
Even if every piece of plastic trash could easily be recycled, that still wouldn’t solve the world’s plastic problem. There are a couple major issues with how recycling currently works that severely limit the usability of recycled materials.
For one thing, recycled plastics inherit all the dyes, flame retardants and other additives that gave each original plastic piece its distinctive look and feel. “The plastic that you actually recover at the end of all this is really a very complex mixture,” says chemist Susannah Scott of the University of California, Santa Barbara. Few manufacturers can use plastic with a random mishmash of properties to make something new.
Plus, recycling breaks some of the chemical bonds in plastic molecules, affecting the strength and consistency of the material. Melting down and remolding plastic is sort of like reheating pizza in the microwave — you get out basically what you put in, just not as good. That limits the number of times plastic can be recycled before it has to be landfilled.
The solution to both problems could lie in a new kind of recycling process, called chemical recycling, which promises to make pure new plastic an infinite number of times. Chemical recycling involves taking plastics apart on the molecular level.
The molecules that make up plastics are called polymers, which are made of smaller monomers. Using heat and chemicals, it is possible to disassemble polymers into monomers, separate those building blocks from dyes and other contaminants, and piece the monomers back together into good-as-new plastic.
“Chemical recycling has really started to emerge as a force, I would say, within the last three or four years,” says University of Pittsburgh’s Beckman. But most chemical recycling techniques are too expensive or energy intensive for commercial use. “It’s not ready for prime time,” he says.
Different plastics require different chemical recycling processes, and some break down more easily than others. “The one that’s farthest along is PET,” Beckman says. “That polymer happens to be easy to take apart.” Several companies are developing methods to chemically recycle PET, including the French company Carbios.
Carbios is testing enzymes produced by microorganisms to break down PET. Researchers at the company described their work on one such enzyme last April in Nature. Microbes normally use the enzyme, called leaf-branch compost cutinase, to decompose the waxy coating on plant leaves. But the cutinase is also good at breaking PET down into its monomers: ethylene glycol and terephthalic acid.
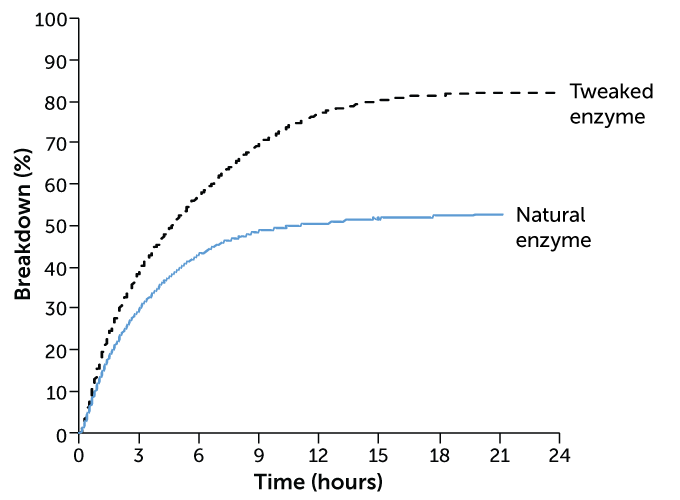
“The enzyme is like a molecular scissor,” says Alain Marty, chief scientific officer at Carbios. But because it evolved to decompose plant matter, not plastic, it’s not perfect. To make the enzyme better at snipping apart PET, “we redesigned what we call the active site of the enzyme,” Marty says. This involved swapping out some of the amino acids along that PET docking site for others.
When the researchers tested their mutant enzyme on colored plastic flakes from PET bottles, applying 3 milligrams of the enzyme per gram of PET, about 90 percent of the plastic broke down in about 10 hours. The original enzyme had maxed out at about 50 percent. Using the terephthalic acid monomers produced in that process, the researchers made new plastic bottles that were just as strong as the originals.
Carbios is now building a plant near Lyon, France, to start chemically recycling PET later this year.
Milder conditions
But other plastics, like polyethylene and polypropylene, are much harder to break down via chemical recycling. Taking apart polyethylene molecules, for instance, requires temperatures over 400° Celsius. At such high heat, the chemistry is chaotic. Plastic molecules break down randomly, generating a complex mixture of compounds that can be burned as fuel but not used to make new materials.
Scott, the UC Santa Barbara chemist, proposes partially breaking down these sturdy plastics in a more controlled way, under milder conditions, to make other kinds of useful molecules. She and colleagues recently came up with a way to transform polyethylene into alkylaromatic compounds, which can be used as biodegradable ingredients in shampoos, detergents and other products. The process involves placing polyethylene inside a reaction chamber set to 280° C, with a catalyst powder containing platinum nanoparticles.
Polyethylene is a long molecule, in which hydrogen atoms are connected to a carbon backbone that can be thousands of carbon atoms long. The platinum is good at breaking carbon-hydrogen bonds, Scott says. “When you do that, you generate hydrogen in the reactor, and the platinum catalyst can use the hydrogen to break the carbon-carbon bonds [in the molecule backbone]. So it actually chops the chain into smaller pieces.”
Since this reaction takes place at a relatively mild 280° C, it happens in an orderly fashion, snapping long polyethylene molecules into shorter chains that are each about 30 carbons long. Those fragments then arrange themselves into the six-sided ring structures characteristic of alkylaromatic compounds.
After 24 hours in the reaction chamber, “most of the products are liquids, and most of the liquids are alkylaromatics,” Scott says. In experiments, about 69 percent of the plastic in a low-density polyethylene bag was converted into liquid. About 55 percent of a high-density polyethylene bottle cap was transformed. The process produces hydrocarbon gases too, which could be used to generate heat to run the reaction at a recycling plant, Scott says.
For now, this is just a lab demo, and like many new recycling strategies, it’s still a long way off from commercialization. And no single upgrade to the recycling pipeline will rid the world of its growing mountains of plastic trash. “We’re going to need a suite of technologies to meet this challenge,” says Daniels, of the REMADE Institute. But each new technology — whether it’s focused on making plastics easier to recycle, or transforming them into more useful materials — could help.