This post was originally published on this site
PHILADELPHIA — Ten patients enrolled in the experimental drug trial, and they were the sickest of the sick.
All had a genetic disorder that cranks up levels of LDL cholesterol in the blood. Known as “bad cholesterol,” LDL cholesterol is infamous for clogging arteries. The patients’ disorder, called heterozygous familial hypercholesterolemia, can lead to severe heart disease at an early age — and death.
Their arteries had been bathing in high LDL cholesterol since birth. In several patients, even typical cholesterol-lowering drugs couldn’t get the levels “even remotely under control,” says Andrew Bellinger, a cardiologist and chief scientific officer at Verve Therapeutics, a Boston-based biotechnology company.
Now, his team has tried a new approach: a genetic medicine called VERVE-101 designed to turn off a cholesterol-raising gene. Using a kind of molecular pencil, the medicine erases one DNA letter and writes in another, inactivating the gene. A single genetic change. A single medication. A potential treatment that lasts a lifetime.
That’s the hope, anyway. Bellinger presented the results of a small clinical trial called heart-1 at the American Heart Association meeting in November. VERVE-101 successfully lowered LDL cholesterol, Bellinger reported. It’s the first time anyone has shown that a DNA spelling change made inside a person’s body could have such an effect. “We can achieve clinically meaningful LDL reductions with a single dose,” he said.
People with familial hypercholesterolemia have lifelong symptoms, so “this whole concept of ‘one and done’ is really amazing,” says Pam Taub, a cardiologist at the University of California, San Diego who was not involved with the trial. These patients must take medications their entire lives. An infused drug like VERVE-101 — designed to alter a person’s DNA — could change treatment strategy.
Taub points out questions about VERVE-101’s safety. One trial patient had a heart attack. Another died due to cardiac arrest. But that death was not related to treatment, Bellinger said.
Moving forward, establishing VERVE-101’s safety is crucial, agreed Karol Watson, a cardiologist at the David Geffen School of Medicine at UCLA who wasn’t involved with the new work. Editing people’s DNA to lower their cholesterol “is a strategy that could be revolutionary, but we have to make sure it’s safe,” she said at the meeting. “You are changing the genome forever.”
Here’s what we know about four key aspects of the new medicine and its history.
VERVE-101 relies on a DNA-modifying protein called a base editor
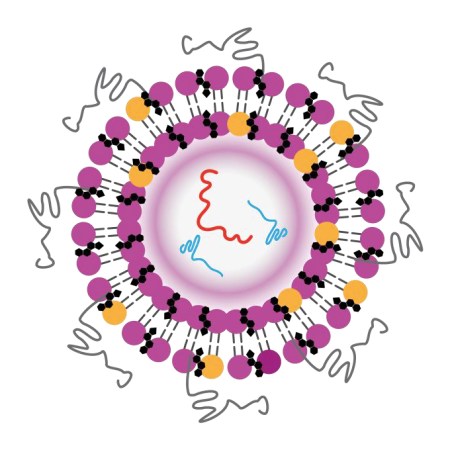
The composition of VERVE-101 is simple. It’s just two types of RNA molecules — molecular cousins of DNA — bundled inside a bubble of fat.
Infused into the bloodstream, the drug travels to the liver and slips into cells. One of the RNA molecules tells cells to build a protein called an adenine base editor. The other acts as a genetic GPS, guiding the editor protein to the correct stretch of DNA.

The experimental gene-editing medicine VERVE-101 packages two kinds of RNA (red and blue, center) inside a lipid nanoparticle, shown in this illustration as dots and squiggles.
The technology is CRISPR 2.0. First generation CRISPR/Cas9 tools act like molecular scissors and can disrupt genes by snipping through DNA’s strands (SN: 8/14/19). Base editors are more like molecular pencils. They edit DNA by performing chemistry on an individual DNA letter, or base, rewriting one for another, creating a new genetic sequence (SN: 10/25/17).
“Base editors actually change a sequence that you choose into a different sequence of your choosing,” says Howard Hughes Medical Institute investigator David Liu, a chemist at Harvard University whose team invented the technology in 2016. In the case of VERVE-101, that sequence is inside the PCSK9 gene, which encodes instructions for manufacturing a protein that raises blood cholesterol levels. Just one edit in a precise location shuts PCSK9 down.
Editing wraps up less than a week after the infusion, and the drug breaks down rapidly, Bellinger said. Both the fat bubble, called a lipid nanoparticle, and its RNA cargo degrade, and within a few weeks, VERVE-101 vanishes from the body. “The only thing that’s left is the DNA change you made to the PCSK9 gene,” he said.
PCSK9 is a tempting target for gene-editing therapy
PCSK9 has been a hot therapeutic target for the last decade or so, says Parag Joshi, a preventive cardiologist at UT Southwestern Medical Center in Dallas who was not part of the trial.
Researchers knew that some people have PCSK9 mutations that switch the gene off. These people tended to have lower levels of LDL cholesterol — and drastically less heart disease, geneticist Helen Hobbs, an HHMI investigator at UT Southwestern Medical Center, and colleagues reported in 2006.
That landmark study pushed the field forward, Joshi says. Suddenly, scientists had proof that people could live healthy lives when PCSK9 was inactivated. That made it “a very attractive drug target,” Joshi says. It suggested that disabling PCSK9 would do no harm — and could even help, by lowering the risk of heart disease.
Typically, the PCSK9 protein breaks down another protein called the LDL receptor. This receptor is one of the good guys; it keeps bad cholesterol in check by snatching it from the blood and transporting it into liver cells for disposal. Without enough LDL receptors, LDL cholesterol levels in the blood ratchet up.
Sekar Kathiresan, a cardiologist and Verve’s CEO and cofounder puts it succinctly: PCSK9 causes disease. “If you turn it off, all you get is health.”
Today, a few existing therapies target PCSK9, including injected antibodies or an RNA-based drug that shuts down production of the protein. Patients should take a daily statin pill to lower LDL cholesterol, Joshi says. But it’s often not enough.
And though the therapies are theoretically effective for people with familial hypercholesterolemia, Kathiresan says, “very few patients are actually on these medications.”
His team thinks that’s because the current approach is just too heavy a burden — asking patients to take daily pills or intermittent injections for decades. “That model doesn’t seem to be working,” Kathiresan says. “And that’s what we’re trying to fix.”
Early VERVE-101 clinical trial results reveal potential benefits — and risks
Kathiresan’s team gave a single IV infusion of VERVE-101 to 10 people with heterozygous familial hypercholesterolemia, most of whom had severe heart disease. In those who received the highest drug doses tested, blood levels of LDL cholesterol dropped sharply, by 39 to 55 percent. And the drop appears long-lasting, Bellinger said. For the patient at the highest dose, LDL cholesterol levels held steady for 180 days after VERVE-101 infused into the bloodstream.
Bellinger called the results “pretty much what we expected and planned,” given the team’s earlier results in nonhuman primates. But the new patient data, though preliminary, places the drug on the precipice of something bigger. “This opens the door for an entirely new way to treat heart disease,” Kathiresan says.
VERVE-101’s utility will ultimately depend on its safety. During the trial, the team spotted some potential red flags. Four patients had minor reactions to the IV infusion, including headache and mild fever. But at the heart meeting, attention hummed over something more severe. A day after the infusion, one patient had a heart attack. Five weeks after the infusion, a different patient died when their heart suddenly stopped beating.
That incident was probably caused by the patients’ underlying heart disease, Kathiresan says. That’s the conclusion reached by an independent data safety monitoring board that investigated the cases, he says.
The heart attack, however, may have been related to the treatment, because it happened so soon after dosing, the monitoring board determined. Kathiresan notes, though, that the patient had been experiencing chest pains prior to the study, something they didn’t mention to the study’s investigators.
These are “very, very sick patients,” says UCSD cardiologist Taub. For future trials with the drug, she thinks such patients should be excluded.
Kathiresan’s team is now planning to enroll patients with less-advanced disease. They’re also going to check for blockages in patients’ arteries, to try and avoid including people at extremely high risk of heart attack.
“This opens the door for an entirely new way to treat heart disease.”
Sekar Kathiresan, Verve Therapeutics CEO and cofounder
In 2024, the company plans to enroll more patients at the two highest doses to determine which dose to move forward. The researchers are also testing a second version of the drug, VERVE-102, which uses a different lipid nanoparticle. Depending on those results, Verve plans to move one of the drugs on to a larger clinical trial in 2025. And if successful in people with familial hypercholesterolemia, the company intends to expand to an even broader group of patients, including those without the genetic disorder.
Developing new medicines is a long road, Kathiresan says. It can take more than a decade for a drug to go from a concept to a medication that doctors can prescribe, he says. Verve started its PCSK9-editing project in 2018. Kathiresan says he hopes to have an approved medication by the end of the decade.
VERVE-101 is one of several base editing drugs currently in clinical trials
One potential side effect of gene-editing therapies is unintentional tweaks to DNA. VERVE-101 targets PCSK9, but what if it strays to a different spot in the genome, asks Anne Goldberg, an endocrinologist at Washington University School of Medicine in St. Louis. The technology “looks really interesting,” she says, “but we need more data.”
A DNA change at the wrong spot could put people at risk of developing cancer. With VERVE-101, Bellinger said, “we think that risk is very low.” Most of the company’s work, he said, goes into demonstrating that “we do not make edits elsewhere in the genome.”
Today’s base editors — including the one in VERVE-101 — are much improved since the early days of the technology, says Harvard’s Liu, who was not involved with the trial. They “have very high on-target editing efficiency while also having minimal off-target editing.”
Currently, five other base-editing clinical trials targeting other diseases, like sickle-cell disease and leukemia, are ongoing. Liu is hopeful that the gene-editing agents will give patients “a completely new lease on life.”
Bellinger said his team thinks of VERVE-101 as a one-time procedure for health, like “molecular surgery without a scalpel.” In theory, it’s possible to reverse the edit made by VERVE-101, but it’s not what he and his colleagues envision.
For cardiologist Donald Lloyd-Jones, who was not involved with the trial, the dream is to offer people with familial hypercholesterolemia a treatment that doesn’t rely on taking a statin pill every single day. A therapy like VERVE-101 “might be something they really consider as an option,” said Lloyd-Jones, of Northwestern University Feinberg School of Medicine in Chicago.
“If we get those safety data, if we get those efficacy data,” he said at the meeting, “I think this would be a very interesting approach to a lifetime fix.”