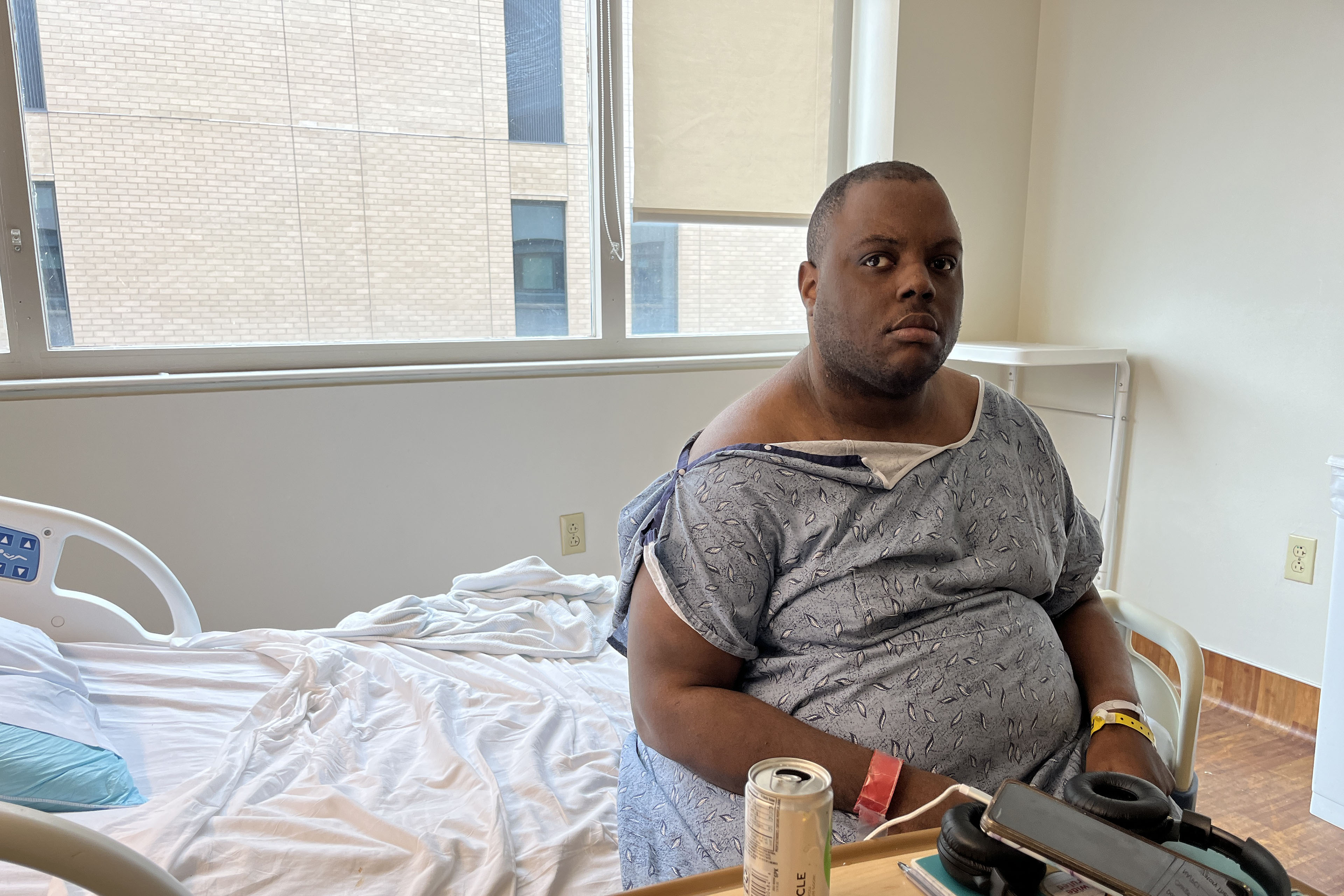
ATLANTA — Lloyd Mills was tired of being stuck in a small, drab hospital room. On a rainy mid-September morning, a small TV attached to a mostly blank white wall played silently. There was nothing in the space to cheer it up — no cards, no flowers.
In February, the 32-year-old with autism, cerebral palsy, and kidney disease was brought to Grady Memorial Hospital from the group home where he had been living because he was having auditory hallucinations and suicidal thoughts, he said.
“Being here is not helping me, mentally, physically, emotionally,” Mills said.
He wanted to return to a group home or some other community setting where he could receive the care he needs without being confined. It’s his legal right. But it took the state agency overseeing his care more than eight months to get that done — and that placement would be short-lived.
Nearly 15 years ago, the U.S. Department of Justice sued Georgia for unnecessarily segregating people with developmental disabilities and mental illness. The state settled the case and agreed to a massive overhaul of the services it offers to that population. Despite hundreds of millions of dollars in investments and some notable improvements, the state’s system of caring for people with developmental disabilities and mental illness still has holes. The gaps often leave people like Mills sequestered in institutional settings and without the proper community supports.
Advocates said those failures continue to violate the rights of Georgians who have been historically marginalized and put their health at risk. “It’s an emergency,” said Susan Walker Goico, director of Atlanta Legal Aid Society’s Disability Integration Project. “Anytime somebody has to live in a segregated setting when they don’t want to, it’s terrible.”
The Americans with Disabilities Act, as clarified in a 1999 U.S. Supreme Court decision, says Mills and other people with disabilities have been legally entitled to receive care at home and in other community settings instead of being unnecessarily confined to places like hospitals and nursing homes.
That decision in Olmstead v. L.C. became the foundation for the lawsuit the Department of Justice levied against Georgia in 2010 that sought to force the state to fix its system.
Later that year, state officials agreed to stop putting people in state hospitals solely because they have developmental disabilities. They also agreed to use Medicaid to pay for people to receive care in the community, and to establish crisis response and housing services for those with mental illness.
The state agreed to make the fixes within five years. Nearly a decade and a half later, it’s still not finished.
Even critics acknowledge Georgia has made considerable improvements in the services it provides for people with developmental disabilities and mental illness. Since the start of the settlement, the state has invested nearly $521 million in community services. And, in late September, a federal judge released the state from many parts of its Olmstead settlement.
However, the DOJ, patient advocates, and even state officials acknowledge more work remains. They say there are many reasons it’s taking so long: the scale of the undertaking, loss of momentum over time, a workforce shortage that has limited appropriate community placements, and a lack of political will.
“The longer it continues, the more you sort of say, ‘Are we serious about solving this problem?’” said Geron Gadd, a senior attorney with the National Health Law Program.
The main challenges won’t be easy to solve without appropriate attention, investments, and commitment from lawmakers, advocates said. In a recent court filing, the state admitted it needs to remove more people with developmental disabilities from psychiatric hospitals, improve case management for people with mental illness, and provide more housing with mental health supports.
That final goal is the “bedrock” of Georgia’s mental health and developmental disability system, Goico said. “You have to have a place to live in order to get your services and to stay out of institutions.”
But people with developmental disabilities and mental illness regularly can’t find appropriate community placements, so they cycle in and out of hospitals and nursing homes, Goico and other observers noted.
In 2010, Georgia launched a housing voucher program for people with mental illness who are chronically homeless, incarcerated, or continually in and out of emergency rooms.
The state agreed to create the capacity to offer vouchers to 9,000 people by July 2015. Currently, only about 2,300 are in the program. Even so, state lawmakers declined to fund additional waivers in next year’s budget, saying they were waiting for an update on Georgia’s compliance with the DOJ settlement.
A legal settlement may dictate that states do certain things, but “the state legislature has to still vote to allocate funds,” said David Goldfarb, former director of long-term supports and services policy at the Arc of the United States, a disability rights organization.
The settlement has resulted in a huge transformation of Georgia’s service system, even though “it’s taking them quite a time to get there,” said Jennifer Mathis, a deputy assistant attorney general with the DOJ’s civil rights division.
For people with developmental disabilities, like Mills, that prolonged arrival means more time confined to hospitals and nursing homes.
Mills said he has had dozens of hospital stays, though none as long as his eight-month stint. “Sometimes it would go from two weeks to a month,” he said in September. “It’s stressful.”
Kevin Tanner, head of Georgia’s Department of Behavioral Health and Developmental Disabilities, noted that the number of people stuck in hospitals had been as high as 30 a day. It’s “down to the teens now,” he said, due in part to the recent opening of two homes for people with developmental disabilities in crisis, with eight beds to serve people statewide.
“No system’s perfect,” Tanner said.
Other states have struggled to achieve compliance. Virginia and North Carolina have been under similar federal oversight since 2012.
But some states have shown it’s possible to make fixes. Delaware entered an Olmstead settlement with the DOJ in 2011 and exited federal oversight five years later. Oregon settled a case in 2015 and achieved compliance in 2022.
In Georgia, a shortage of housing for people with developmental disabilities and mental illness has been exacerbated by the shuttering of home and community service providers in recent years, said Lisa Reisman, owner of Complete Care at Home, which offers home medical care to older adults and people with disabilities.
Many service providers blamed the shortage of home and community services on Georgia’s low Medicaid reimbursement rates, which have made it hard for providers to keep workers. Years of low rates “decimated the infrastructure,” said Ryan Whitmire, president of Developmental Disabilities Ministries of Georgia.
Reisman said she has had to turn down placement requests from the state because she couldn’t accommodate them. In those situations, she said, a state official said service providers would sometimes drop off clients at ERs because they “were out of money and they didn’t know where to put them.”
Service providers, including Whitmire, said nurses and other caregivers often leave for higher-paying jobs in fast food or retail.
This year, state lawmakers appropriated more than $106 million to increase Medicaid rates for mental health and developmental disability service providers. Some of those rates hadn’t been raised since 2008.
State lawmakers also recently passed a bill that would require a study every four years of rates it pays providers — though it would still be up to lawmakers to increase payments.
Not only was Lloyd Mills’ extended time in the hospital hard mentally and physically, it also made him lose his Medicaid coverage, said his representatives from the Georgia Advocacy Office, a nonprofit that represents people with disabilities.
Because he was in a hospital, he was unable to spend his monthly Supplemental Security Income payments, which accumulated until he had too much money to keep his health coverage.
In late October, eight months after his hospital stay began, the state moved him to a group home in Macon, about 85 miles southeast of Atlanta. In the days before his move, Mills said he was ready to start his next chapter.
“I’m just ready to live my life, and I don’t plan on ever coming back here again,” he said.
But his stay was short. In mid-November, after just a few weeks of living at the group home, Mills ended up back in a hospital. His advocates worry he won’t be heading to a community placement anytime soon.
This article was produced by KFF Health News, a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.