James Lemons, 39, wants the bullet removed from his thigh so he can go back to work.
Sarai Holguin, a 71-year-old woman originally from Mexico, has accepted the bullet lodged near her knee as her “compa” — a close friend.
Mireya Nelson, 15, was hit by a bullet that went through her jaw and broke her shoulder, where fragments remain. She’ll live with them for now, while doctors monitor lead levels in her blood for at least two years.
Nearly three months after the Kansas City Chiefs Super Bowl parade shooting left at least 24 people injured, recovery from those wounds is intensely personal and includes a surprising gray area in medicine: whether the bullets should be removed.
Medical protocol offers no clear answer. A 2016 survey of surgeons found that only about 15% of respondents worked at medical facilities that had policies on bullet removal. Doctors in the U.S. often leave bullets buried deep in a person’s body, at least at first, so as not to cause further trauma.
But as gun violence has emerged as a public health epidemic, some researchers wonder if that practice is best. Some of the wounded, like James Lemons, are left in a precarious place.
“If there’s a way to get it out, and it’s safely taken out, get it out of the person,” Lemons said. “Make that person feel more secure about themselves. And you’re not walking around with that memory in you.”
Lemons, Holguin, and Nelson are coping in very different ways.
Pain Became a Problem
Three days after the Chiefs won the Super Bowl, Lemons drove the 37 miles from Harrisonville, Missouri, to downtown Kansas City to celebrate the victory. The warehouse worker was carrying his 5-year-old daughter, Kensley, on his shoulders when he felt a bullet enter the back of his right thigh.

Gunfire erupted in the area packed with revelers, prosecutors later said, after a “verbal confrontation” between two groups. Detectives found “multiple 9mm and .40 caliber spent shell casings” at the scene. Lemons said he understood immediately what was happening.
“I know my city. We’re not shooting off fireworks,” he said.
Lemons shielded Kensley’s face as they fell to the ground so she wouldn’t hit the concrete. His first thought was getting his family — also including his wife, Brandie; 17-year-old daughter, Kallie; and 10-year-old son, Jaxson — to safety.
“I’m hit. But don’t worry about it,” Lemons recalled telling Brandie. “We gotta go.”
He carried Kensley on his shoulders as the family walked a mile to their car. His leg bled through his pants at first then stopped, he said. It burned with pain. Brandie insisted on driving him to the hospital but traffic was at a standstill so she put on her hazard lights and drove on the wrong side of the road.
“She’s like: ‘I’m getting you to a hospital. I’m tired of people being in my way,’” Lemons recalled. “I’ve never seen my wife like that. I’m looking at her like, ‘That’s kinda sexy.’”
Lemons clapped and smiled at his wife, he said, to which she replied, “What are you smiling for? You just got shot.” He stayed in quiet admiration until they were stopped by a sheriff, who summoned an ambulance, Lemons said.
He was taken to the emergency room at University Health, which admitted 12 patients from the rally, including eight with gunshot wounds. Imaging showed the bullet barely missed an artery, Lemons said. Doctors cleansed the wound, put his leg in a brace, and told him to come back in a week. The bullet was still in his leg.
“I was a little baffled by it, but I was like, ‘OK, whatever, I’ll get out of here,’” Lemons recalled.
When he returned, doctors removed the brace but explained they often leave bullets and fragments in the body — unless they grow too painful.
“I get it, but I don’t like that,” Lemons said. “Why wouldn’t you take it out if you could?”
University Health spokesperson Leslie Carto said the hospital can’t comment on individual patient care because of federal privacy laws.
Surgeons typically do remove bullets when they encounter them during surgery or they are in dangerous locations, like in the spinal canal or risking damage to an organ, said Brendan Campbell, a pediatric surgeon at Connecticut Children’s.
Campbell also chairs the Injury Prevention and Control Committee of the American College of Surgeons’ Committee on Trauma, which works on firearm injury prevention.
LJ Punch, a trauma surgeon by training and the founder of the Bullet Related Injury Clinic in St. Louis, said the origins of trauma care also help explain why bullets are so often left.
“Trauma care is war medicine,” Punch said. “It is set to be ready at any moment and any time, every day, to save a life. It is not equipped to take care of the healing that needs to come after.”
In the survey of surgeons, the most common reasons given for removing a bullet were pain, a palpable bullet lodged near the skin, or an infection. Far less common were lead poisoning and mental health concerns such as post-traumatic stress disorder and anxiety.
What patients wanted also affected their decisions, the surgeons said.

Lemons wanted the bullet out. The pain it caused in his leg radiated up from his thigh, making it difficult to move for more than an hour or two. Working his warehouse job was impossible.
“I gotta lift 100 pounds every night,” Lemons recalled telling his doctors. “I gotta lift my child. I can’t work like this.”
He has lost his income and his health insurance. Another stroke of bad luck: The family’s landlord sold their rental home soon after the parade, and they had to find a new place to live. This house is smaller, but it was important to keep the kids in the same school district with their friends, Lemons said in an interview in Kensley’s pink bedroom, the quietest spot to talk.
They’ve borrowed money and raised $6,500 on GoFundMe to help with the deposit and car repairs, but the parade shooting has left the family in a deep financial hole.
Without insurance, Lemons worried he couldn’t afford to have the bullet removed. Then he learned his surgery would be paid for by donations. He set up an appointment at a hospital north of the city, where a surgeon took measurements on his X-ray and explained the procedure.
“I need you to be involved as much as I’m going to be involved,” he remembered being told, “because — guess what — this ain’t my leg.”
The surgery is scheduled for this month.
‘We Became Friends’
Sarai Holguin isn’t much of a Chiefs fan, but she agreed to go to the rally at Union Station to show her friend the best spot to see the players on stage. It was an unseasonably warm day, and they were standing near an entrance where lots of police were stationed. Parents had babies in strollers, kids were playing football, and she felt safe.
A little before 2 p.m., Holguin heard what she thought were fireworks. People started running away from the stage. She turned to leave, trying to find her friend, but felt dizzy. She didn’t know she’d been shot. Three people quickly came to her aid and helped her to the ground, and a stranger took off his shirt and made a tourniquet to put on her left leg.
Holguin, a native of Puebla, Mexico, who became a U.S. citizen in 2018, had never seen so much chaos, so many paramedics working under such pressure. They were “anonymous heroes,” she said.
She saw them working on Lisa Lopez-Galvan, a well-known DJ and 43-year-old mother of two. Lopez-Galvan died at the scene, and was the sole fatality at the parade. Holguin was rushed to University Health, about five minutes from Union Station.

There doctors performed surgery, leaving the bullet in her leg. Holguin awoke to more chaos. She had lost her purse, along with her cellphone, so she couldn’t call her husband, Cesar. She had been admitted to the hospital under an alias — a common practice at medical centers to begin immediate care.
Her husband and daughter didn’t find her until about 10 p.m. — roughly eight hours after she’d been shot.
“It has been a huge trauma for me,” Holguin said through an interpreter. “I was injured and at the hospital without doing anything wrong. [The rally] was a moment to play, to relax, to be together.”
Holguin was hospitalized for a week, and two more outpatient surgeries quickly followed, mostly to remove dead tissue around the wound. She wore a wound VAC, or vacuum-assisted closure device, for several weeks and had medical appointments every other day.
Campbell, the trauma surgeon, said wound VACs are common when bullets damage tissue that isn’t easily reconstructed in surgery.
“It’s not just the physical injuries,” Campbell said. “Many times it’s the emotional, psychological injuries, which many of these patients take away as well.”
The bullet remains near Holguin’s knee.

“I’m going to have it for the rest of my life,” she said, saying she and the bullet became “compas,” close friends.
“We became friends so that she doesn’t do any bad to me anymore,” Holguin said with a smile.
Punch, of the Bullet Related Injury Clinic in St. Louis, said some people like Holguin are able to find a way to psychically live with bullets that remain.
“If you’re able to make a story around what that means for that bullet to be in your body, that gives you power; that gives you agency and choice,” Punch said.
Holguin’s life changed in an instant: She’s using a walker to get around. Her foot, she said, acts “like it had a stroke” — it dangles, and it’s difficult to move her toes.
The most frustrating consequence is that she cannot travel to see her 102-year-old father, still in Mexico. She has a live camera feed on her phone to see him, but that doesn’t offer much comfort, she said, and thinking about him brings tears.
She was told at the hospital that her medical bills would be taken care of, but then lots of them came in the mail. She tried to get victim assistance from the state of Missouri, but all the forms she had were in English, which made them difficult to comprehend. Renting the wound VAC alone cost $800 a month.
Finally she heard that the Mexican Consulate in Kansas City could help, and the consul pointed her to the Jackson County Prosecutor’s Office, with which she registered as an official victim. Now all of her bills are being paid, she said.
Holguin isn’t going to seek mental health treatment, as she believes one must learn to live with a given situation or it will become a burden.
“I have processed this new chapter in my life,” Holguin said. “I have never given up and I will move on with God’s help.”

‘I Saw Blood on My Hands’
Mireya Nelson was late to the parade. Her mother, Erika, told her she should leave early, given traffic and the million people expected to crowd into downtown Kansas City, but she and her teenage friends ignored that advice. The Nelsons live in Belton, Missouri, about a half hour south of the city.
Mireya wanted to hold the Super Bowl trophy. When she and her three friends arrived, the parade that had moved through downtown was over and the rally at Union Station had begun. They were stuck in the large crowd and quickly grew bored, Mireya said.
Getting ready to leave, Mireya and one of her friends were trying to call the driver of their group, but they couldn’t get cell service in the large crowd.
Amid the chaos of people and noise, Mireya suddenly fell.
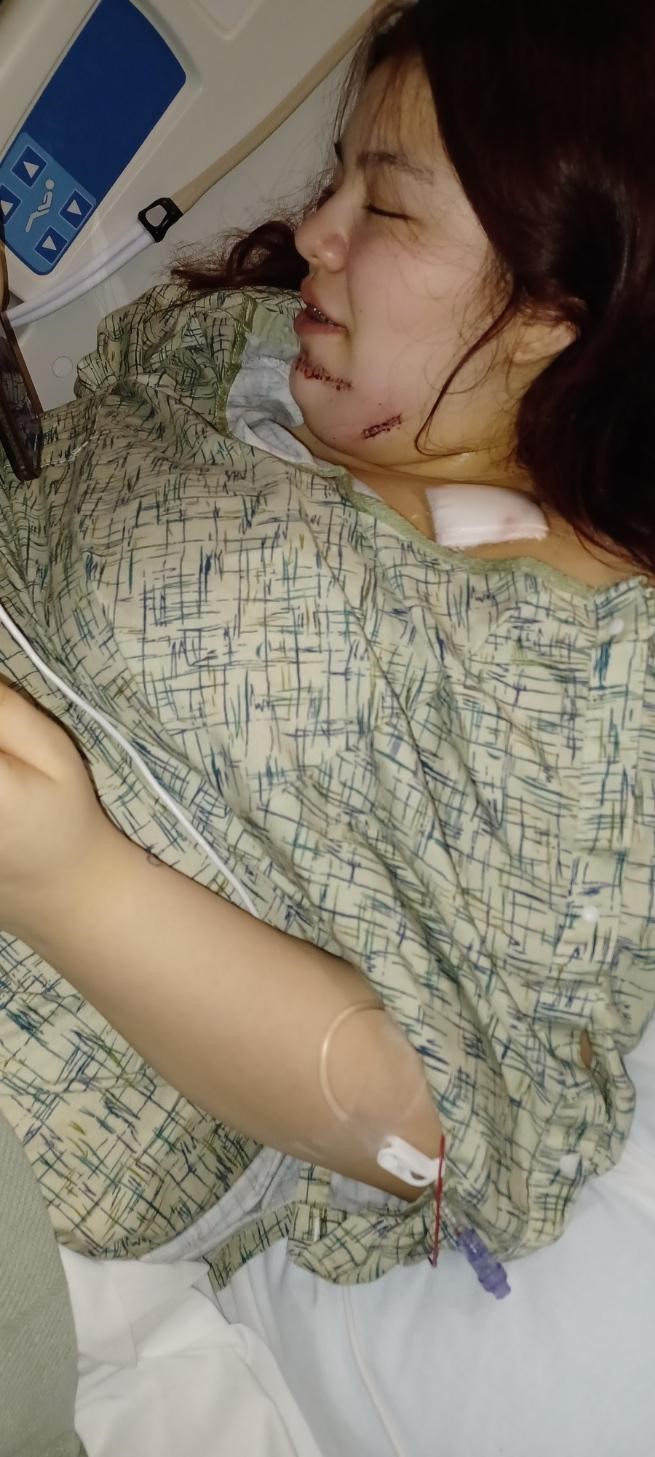
“I saw blood on my hands. So then I knew I got shot. Yeah, and I just crawled to a tree,” Mireya said. “I actually didn’t know where I got shot at, at first. I just saw blood on my hands.”
The bullet grazed Mireya’s chin, shot through her jaw, broke her shoulder, and left through her arm. Bullet fragments remain in her shoulder. Doctors decided to leave them because Mireya had already suffered so much damage.
Mireya’s mother supports that decision, for now, noting they were just “fragments.”
“I think if it’s not going to harm her the rest of her life,” Erika said, “I don’t want her to keep going back in the hospital and getting surgery. That’s more trauma to her and more recovery time, more physical therapy and stuff like that.”
Bullet fragments, particularly ones only skin-deep, often push their way out like splinters, according to Punch, although patients aren’t always told about that. Moreover, Punch said, injuries caused by bullets extend beyond those with damaged tissue to the people around them, like Erika. He called for a holistic approach to recover from all the trauma.
“When people stay in their trauma, that trauma can change them for a lifetime,” Punch said.
Mireya will be tested for lead levels in her blood for at least the next two years. Her levels are fine now, doctors told the family, but if they get worse she will need surgery to remove the fragments, her mother said.
Campbell, the pediatric surgeon, said lead is particularly concerning for young children, whose developing brains make them especially vulnerable to its harmful effects. Even a tiny amount of lead — 3.5 micrograms per deciliter — is enough to report to state health officials, according to the Centers for Disease Control and Prevention.

Mireya talks about cute teenage boys’ being “fine” but also still wears Cookie Monster pajamas. She appears confused by the shootings, by all the attention at home, at school, from reporters. Asked how she feels about the fragments in her arm, she said, “I don’t really care for them.”
Mireya was on antibiotics for 10 days after her hospital stay because doctors feared there was bacteria in the wound. She has had physical therapy, but it’s painful to do the exercises. She has a scar on her chin. “A dent,” she said, that’s “bumpy.”
“They said she was lucky because if she wouldn’t have turned her head in a certain way, she could be gone,” Erika said.
Mireya faces a psychiatric evaluation and therapy appointments, though she doesn’t like to talk about her feelings.
So far, Erika’s insurance is paying the medical bills, though she hopes to get some help from the United Way’s #KCStrong fund, which raised nearly $1.9 million, or a faith-based organization called Unite KC.
Erika doesn’t want a handout. She has a job in health care and just got a promotion.
The bullet has changed the family’s life in big ways. It is part of their conversation now. They talk about how they wish they knew what kind of ammunition it was, or what it looked like.
“Like, I wanted to keep the bullet that went through my arm,” Mireya said. “I want to know what kind of bullet it was.” That brought a sigh from her mom, who said her daughter had watched too many episodes of “Forensic Files.”
Erika beats herself up about the wound, because she couldn’t protect her daughter at the parade.
“It hits me hard because I feel bad because she begged me to get off work and I didn’t go there because when you have a new position, you can’t just take off work,” Erika said. “Because I would have took the bullet. Because I would do anything. It’s mom mode.”
This article was produced by KFF Health News, a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.