This post was originally published on this site
Christopher Marks noticed an immediate improvement when his doctor prescribed him the Type 2 diabetes medication Mounjaro last year. The 40-year-old truck driver from Kansas City, Missouri, said his average blood sugar reading decreased significantly and that keeping it within target range took less insulin than before.
But when his doctor followed the typical prescribing pattern and increased his dose of Mounjaro — a drug with a wholesale list price of more than $1,000 a month — Marks’ health insurer declined to pay for it.
Marks had Cigna insurance that he purchased on the federal health insurance marketplace, healthcare.gov. After two appeals over a month and a half, Cigna agreed to cover the higher dose. A few months later, he said, when it was time to up his dose once more, he was denied again. By November, he decided it wasn’t worth sparring with Cigna anymore since the insurer was leaving the marketplace in Missouri at the start of this year. He decided to stay on the lower dose until his new insurance kicked in.
“That is beyond frustrating. People shouldn’t have to be like, ‘It’s not worth the fight to get my medical treatment,’” Marks said.
The process Marks encountered is called “prior authorization,” or sometimes “pre-certification,” a tool insurers say they use to rein in costs and protect patients from unnecessary or ineffective medical treatment. But the practice has prompted backlash from patients like Marks, as well as groups representing medical professionals and hospitals that say prior authorization can interfere with treatment, cause medical provider burnout, and increase administrative costs.
In January, the Biden administration announced new rules to streamline the process for patients with certain health plans, after attempts stalled out in Congress, including a bill that passed the House in 2022. But states are considering prior authorization bills that go even further. Last year, lawmakers in 29 states and Washington, D.C., considered some 90 bills to limit prior authorization requirements, according to the American Medical Association, with notable victories in New Jersey and Washington, D.C. The physicians association expects more bills this year, many with provisions spelled out in model legislation the group drafted.
In 2018, health insurers signed a consensus statement with various medical facility and provider groups that broadly laid out areas for improving the prior authorization process. But the lack of progress since then has shown the need for legislative action, said Jack Resneck Jr., past president of the AMA and a current trustee.
“They have not lived up to their promises,” Resneck said.

Resneck, a California dermatologist, emphasized pending bills in Indiana, Massachusetts, North Carolina, Oklahoma, and Wyoming that include several policies backed by the AMA, including quicker response times, requirements for public reporting of insurers’ prior authorization determinations, and programs to reduce the volume of requests, sometimes called “gold carding.” Legislation has come from both Democratic and Republican lawmakers, and some is bipartisan, as in Colorado.
In Missouri, legislation introduced by Republican state Rep. Melanie Stinnett aims to establish one of those gold carding programs for treatment and prescriptions. Stinnett said she regularly was frustrated by prior authorization hurdles in her work as a speech pathologist before joining the legislature in 2023.
“The stories all kind of look similar: It’s a big fight to get something done on the insurance side for approval,” Stinnett said. “Then sometimes, even after all of that fight, it feels like it may have not been worthwhile because some people then have a change at the beginning of the year with their insurance.”
Under her bill, a medical provider’s prior authorization requests during a six-month evaluation period would be reviewed. After that period, providers whose requests were approved at least 90% of the time would be exempt from having to submit requests for the next six months. The exemptions would also apply to facilities that meet that threshold. Then, she said, they would need to continue meeting the threshold to keep the “luxury” of the exemption.
Five states have passed some form of gold carding program: Louisiana, Michigan, Texas, Vermont, and West Virginia. The AMA is tracking active gold carding bills in 13 states, including Missouri.
A 2022 survey of 26 health insurance plans conducted by the industry trade group AHIP found that just over half of those plans had used a gold carding program for medical services while about a fifth had done so for prescriptions. They gave mixed reviews: 23% said patient safety improved or stayed the same, while 20% said the practice increased costs without improving quality.
The new federal prior authorization rules finalized by the Centers for Medicare & Medicaid Services stop short of gold carding and don’t address prior authorizations for prescription drugs, like Marks’ Mounjaro prescription. Beginning in 2026, the new rules establish response time frames and public reporting requirements — and ultimately will mandate an electronic process — for some insurers participating in federal programs, such as Medicare Advantage or the health insurance marketplace. Manual submissions accounted for 39% of prior authorization requests for prescriptions and 60% of those for medical services, according to the 2022 insurance survey.
In Missouri, state and national organizations representing doctors, nurses, social workers, and hospitals, among others, back Stinnett’s bill. Opposition to the plan comes largely from pharmacy benefit managers and the insurance industry, including the company whose prior authorization process Marks navigated last year. A Cigna Healthcare executive submitted testimony saying the company’s experience showed gold card policies “increase inappropriate care and costs.”
The St. Louis Area Business Health Coalition, which represents dozens of employers that purchase health insurance for employees, also opposes the bill. Members of the coalition include financial services firm Edward Jones, coal company Peabody Energy, and aviation giant Boeing, as well as several public school districts and the St. Louis city and county governments.
Louise Probst, the coalition’s executive director, said the prior authorization process has issues but that the coalition would prefer that a solution come from insurers and providers rather than a new state law.
“The reason I hate to see things just set in stone is that you lose the flexibility and the nuance that could be helpful to patients,” Probst said.
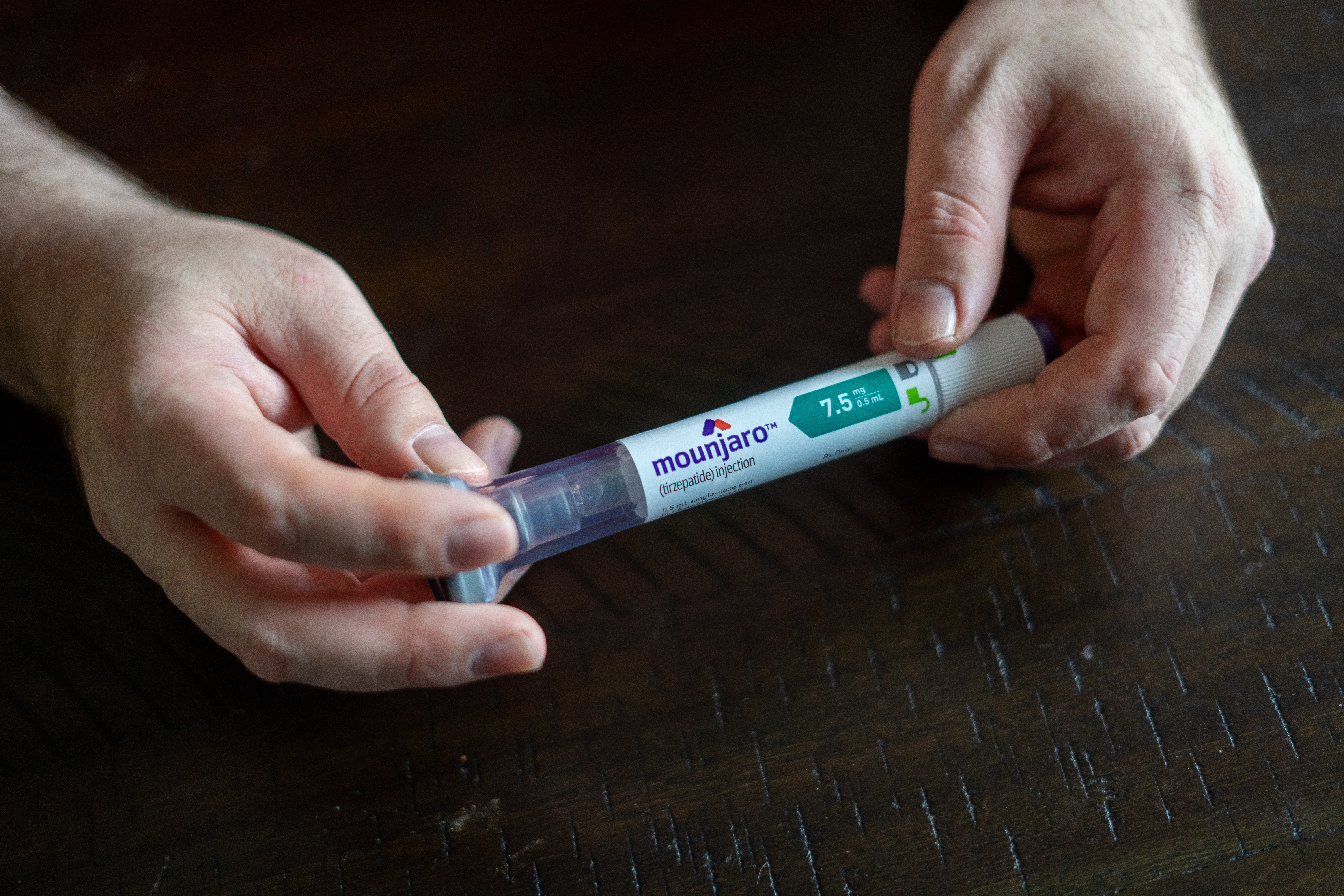
On the other side of the state, Marks purchased insurance for this year on the federal marketplace from Blue Cross and Blue Shield of Kansas City. In January, his doctor re-prescribed the higher dose of Mounjaro that Cigna had declined to cover. A little over a week later, Marks said, his new insurance approved the higher dose “without any fuss.”
Cigna spokesperson Justine Sessions said the company uses prior authorizations for popular drugs such as Mounjaro to help ensure patients get the right medications and dosages.
“We strive to make authorizations quickly and correctly, but in Mr. Marks’ case, we fell short and we greatly regret the stress and frustration this caused,” she said. “We are reviewing this case and identifying opportunities for improvement to ensure this does not happen in the future.”
Marks’ aim with this higher dose of Mounjaro is to get off his other diabetes medications. He particularly hopes to stop taking insulin, which for him requires multiple injections a day and carries a risk of dangerous complications from low blood sugar.
“I don’t really use the word ‘life-changing,’ but it kind of is,” Marks said. “Getting off insulin would be great.”
Do you have an experience with prior authorization you’d like to share? Click here to tell your story.
This article was produced by KFF Health News, formerly known as Kaiser Health News (KHN), a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.